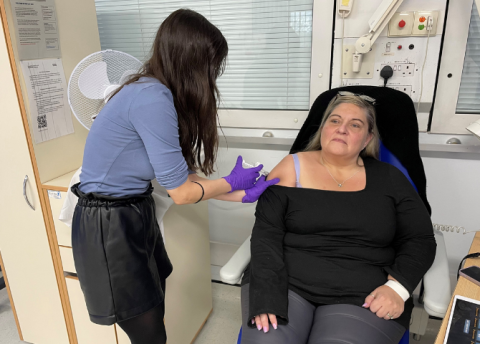
A new treatment to help people with severe asthma which cannot be controlled by inhalers and steroids is being given to patients at Royal Brompton Hospital following the success of an international clinical trial led by consultants at the same hospital.
Tezepelumab, is made up of human antibodies that suppress the specific part of the body’s immune system that leads to unnecessary inflammation, causing severe asthma attacks. It is part of a new generation of drugs called ‘biologics’.
The new drug, given as an injection, has been found to reduce the number of asthma attacks and admissions to hospital or A&E in patients with severe asthma. In the clinical trial published in 2021, 43.8% of patients on tezepelumab experienced an asthma attack compared to 60.1% of patients who did not receive the drug. Patients on tezepelumab had a 79% lower risk of being admitted to hospital or A&E than patients not on the treatment.
People with asthma classed as ‘severe’ have symptoms which are difficult to control even with high doses of medicines. It is the most serious and life-threatening type of asthma and is classed as a disability under the Equality Act. Approximately 200,000 people in the UK live with severe asthma.
Claire Newman from Bexley, a 48-year-old patient who received her first dose of tezepelumab at Royal Brompton Hospital, said: “My asthma really limits what I can do, simple things like just moving around the flat is a real struggle. Housework, which I used to be able to do without a problem, is now just off limits because it can trigger asthma attacks. I find it really frustrating and it affects everything I do.
“I had tried previous biologics, but this is the one I have been waiting for. My consultant was one of the people who developed this treatment at the Brompton and he is hopeful this could be the treatment that could make a real difference for me, so I’m really excited.”
The treatment is administered as a monthly injection over the patient’s lifetime with the patients’ receiving guidance from asthma clinical nurse specialists so they can administer the injections at home instead of having to make frequent visits to the hospital.
Dr Pujan Patel, a consultant respiratory physician at Royal Brompton Hospital and clinical lead for the hospital’s severe asthma service, said: “Patients with severe asthma have tried everything to control their debilitating condition. Where most people with asthma can control their symptoms with preventative and rescue inhalers, the patients I see in our severe asthma clinic are on long, repetitive courses of steroids which can help in the short term, but which have their own long term side effects including weight gain, skin thinning, brittle bones and stomach ulcers.
“During the clinical trial which has led to tezepelumab being used in the clinic today, nearly 70% of patients reported a response to the medication. Whether this was needing to use their rescue inhaler less frequently or being able to do more activities or not needing to use steroids at all, many of the patients describe the drug as ‘life-changing’.
“We expect to see the patients who are being given tezepelumab in our clinic to respond to the treatment within six months. I am optimistic and hopeful that this treatment can offer them a chance to lead healthy, normal lives once again.”
Patients at Royal Brompton Hospital and Guy’s Hospital, both a part of Guy’s and St Thomas’ NHS Foundation Trust, are the first in London to receive tezepelumab as a treatment for severe asthma.
The severe asthma service at Royal Brompton Hospital is the UK’s largest standalone severe asthma service and looks after approximately 1,300 patients a year with severe asthma on asthma biologic treatments, and anticipates that around 100 of the most severe patients will be eligible to receive the new treatment in the first instance.
Michelle Ramsey from Gravesend, a 57-year-old patient who received her first dose of tezepelumab at Royal Brompton Hospital, said: “My asthma means that I can’t do an awful lot. I have difficulty breathing, I can’t walk far, it’s just so restrictive and I feel embarrassed about it. I am hoping this new drug will be life-changing for me. I just want to do everyday things without triggering my asthma, and I cannot wait to be able experience life and breathe freely.”
Draft guidance published by the National Institute for Health and Care Excellence (NICE) recommended the use of tezepelumab as an “additional maintenance treatment” for people over 12 with severe asthma.
Contact information
Media enquiries
Phone: 020 7188 5577
Email: press@gstt.nhs.uk